Background: Venetoclax (Ven) in combination with hypomethylating agents is approved for treatment in patients (pts) with newly diagnosed (ND) acute myeloid leukemia (AML) who are ≥75 years old or unfit for intensive chemotherapy (IC), based on the longer median overall survival (OS) with Ven+azacitidine (Aza) compared with Aza in combination with placebo in the Phase III VIALE-A study (NCT02993523). Several studies are now evaluating Ven-based regimens in pts eligible for IC, for example Ven+Aza compared with IC (NCT04801797). Additionally, Ven in combination with cladribine (CLAD) and low-dose cytarabine (LDAC) is currently under investigation in a Phase II single-arm study (NCT03586609) amongst older pts (aged ≥60 years) with ND AML. An interim analysis of this trial evaluated 60 pts where complete remission (CR) and CR with incomplete count recovery (CRi) was observed in 93% of pts, and the median OS, overall and by transplant status, was not reached; 1-year OS was 72.9% and 2-year OS was 63.5% (Kadia et al. J Clin Oncol 2022). The aim of this study is to better understand treatment outcomes amongst older pts (aged ≥60 years) with ND AML receiving IC in a routine clinical care setting in the United States (US).
Methods: This was a retrospective cohort study using the Flatiron Health electronic health record-derived, US nationwide, de-identified database. Pts aged ≥60 years with ND AML who initiated IC within 60 days of AML diagnosis (Dx) between January 1, 2014, and November 30, 2022, with ≥2 visits recorded within 3 months of AML Dx, and ≥3 months of follow-up before study end date (February 28, 2023) were included. Clinical trial participants and pts with acute promyelocytic leukemia or core binding factor were excluded. Descriptive analysis on patient characteristics was conducted. The proportion of pts who achieved real-world response (defined as <5% bone marrow [BM] blasts), real-world CR/CRi (defined as <5% BM blast with either platelet count <100 x 10 9/L or absolute neutrophil count <1000 x 10 9/L) and received a stem cell transplant (SCT) post-remission were reported. Thirty-day mortality, 60-day mortality, 1-year real-world OS (rwOS), and 2-year rwOS were evaluated using Kaplan-Meier (KM) analyses. The starting points for KM analyses were IC treatment initiation (all pts), SCT (pts who underwent SCT post-remission), and remission (pts who did not undergo SCT post-remission). Pts were censored at the last activity date or SCT date (whichever occurred earliest) for the overall patient cohort, and the last activity date for pts with or without SCT post-remission.
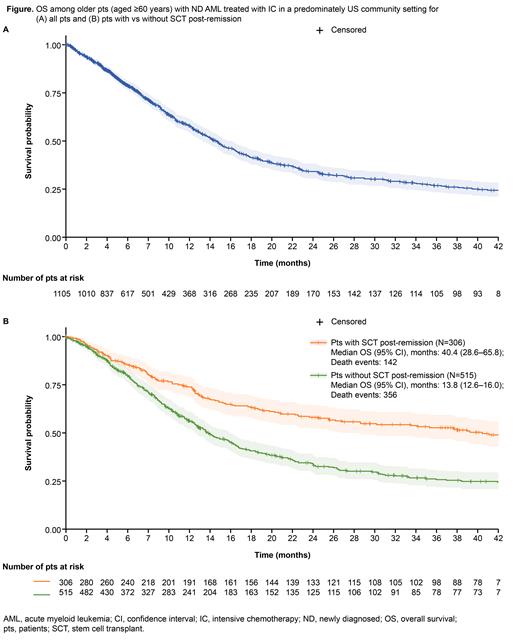
Results: A total of 1105 pts met the study criteria. The median (IQR) age was 67 (64-71) years, 57% were male, 27% had secondary AML, 48% had BM blast >50% at Dx, and 66% were treated in the community setting. Excluding 340 pts with unknown cytogenetic and molecular data, 19%, 32%, and 48% of pts had European LeukemiaNet 2017 favorable, intermediate, and adverse risk, respectively. At a median (IQR) follow-up of 13 (6-28) months from AML Dx, 74% (n=821) of pts achieved real-world response, including 66% (n=724) who achieved real-world CR/CRi. For all pts, the 30-day mortality was 3%, 60-day mortality was 6%, 1-year rwOS was 58%, 2-year OS was 34%, and the median rwOS was 14.3 months ( Figure A). A total of 37% (n=306/821) of pts underwent SCT post-remission; 98% (n=300/306) underwent allogeneic-SCT and 2% (n=6/306) underwent autologous-SCT. The median rwOS was 40.4 months for pts undergoing SCT post-remission, and 13.8 months for pts without SCT post-remission ( Figure B).
Conclusions: In this large real-world data study, we observed older pts (aged ≥60 years) with ND AML receiving IC treatment had a median OS of <14 months and <35% of pts remained alive 2 years after IC initiation. Findings from this study suggest the need for other novel treatment options, including Ven based regimens, to improve OS in older pts (aged ≥60 years) with ND AML who are eligible for IC. Future studies are warranted to conduct randomized controlled trials to comparatively evaluate IC vs other novel treatment options in older pts (aged ≥60 years) with ND AML, or conduct the appropriate statistical adjustment to minimize confounding in indirect treatment comparisons from different studies.
Disclosures
Kadia:Hikma Pharmaceuticals: Speakers Bureau; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Agios: Consultancy; Genzyme: Honoraria; Genentech: Consultancy, Research Funding; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; Pfizer: Consultancy, Research Funding; Janssen Research and Development: Research Funding; Servier: Consultancy; Cellenkos Inc.: Research Funding; Liberum: Consultancy; Delta-Fly Pharma, Inc.: Research Funding; Iterion: Research Funding; Novartis: Consultancy; Regeneron Pharmaceuticals: Research Funding; Pulmotect, Inc.: Consultancy, Research Funding; Cure: Speakers Bureau; Celgene: Research Funding; Glycomimetics: Research Funding; Astellas Pharma Global Development: Research Funding; AstraZeneca: Research Funding; Amgen, Inc.: Research Funding; GenFleet Therapeutics: Research Funding; SELLAS Life Sciences Group: Research Funding; Sanofi-Aventis: Consultancy; Cyclacel: Research Funding; Ascentage Pharma Group: Research Funding; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria. Ma:Genentech, Inc.: Current Employment, Current equity holder in private company. Patel:Genentech, Inc. (F. Hoffmann-La Roche Ltd): Current equity holder in publicly-traded company; GlaxoSmithKline (via University of North Carolina - Chapel Hill), Regeneron: Ended employment in the past 24 months; Genentech, Inc.: Current Employment. Wang:F. Hoffmann-La Roche Ltd (stock and options as an employee): Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Yellow-Duke:Genentech, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Montez:Genentech, Inc./F. Hoffmann-La Roche Ltd: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company. Bui:AbbVie Inc: Current Employment, Current holder of stock options in a privately-held company. Ravandi:Amgen: Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Biomea fusion: Honoraria, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Prelude: Research Funding; Xencor: Research Funding; Astellas: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal